Chapter 6 Section 1 Atoms Elements And Compounds
In 1913 chemistry and physics were topsy-turvy. Electronic Configurations of the d-Block Elements.
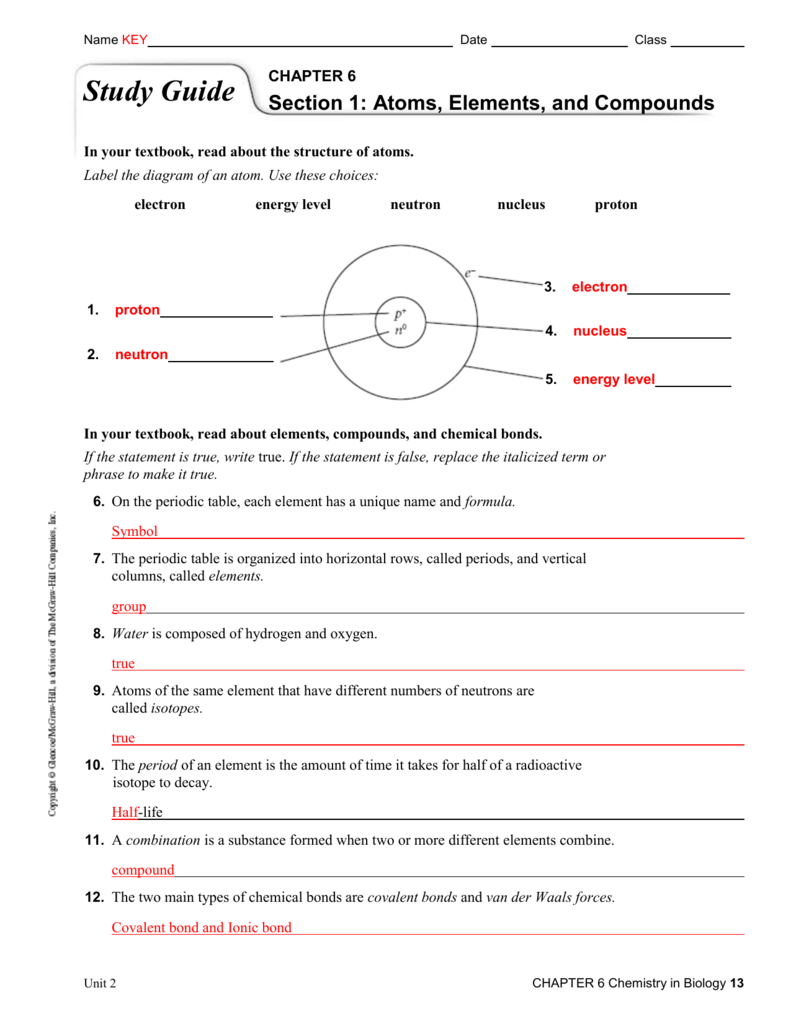
Chapter 6 Study Guide Key
Modification of work.
. It is the smallest constituent of an element that exhibits its properties. 25 Organic Compounds Essential to Human Functioning. If we add 01 moles 60210 22 molecules of HCl to a solution to make a liter it will have 01 moles of H and 01 moles of Cl- or 60210 22 molecules of each.
These are neither created nor destroyed in a chemical reaction. What is a natural product chemistry and why should we be interested in studying it. Milk plant exudates and other natural materials that were once found in living.
Atoms of group 16 gain two electrons and form ions with a 2 charge and so on. Molecules can be formed by two or more atoms in a chemical bonding. 115 Muscles of the Pectoral Girdle and Upper Limbs.
The modern statement of this relationship the periodic law is as follows. 111 Interactions of Skeletal Muscles Their Fascicle Arrangement and Their Lever Systems. Compounds are formed when atoms of different elements combine in a fixed ratio.
114 Axial Muscles of the Abdominal Wall and Thorax. The Building Blocks of Matter. Chapter 5 Introduction to Organic Chemistry.
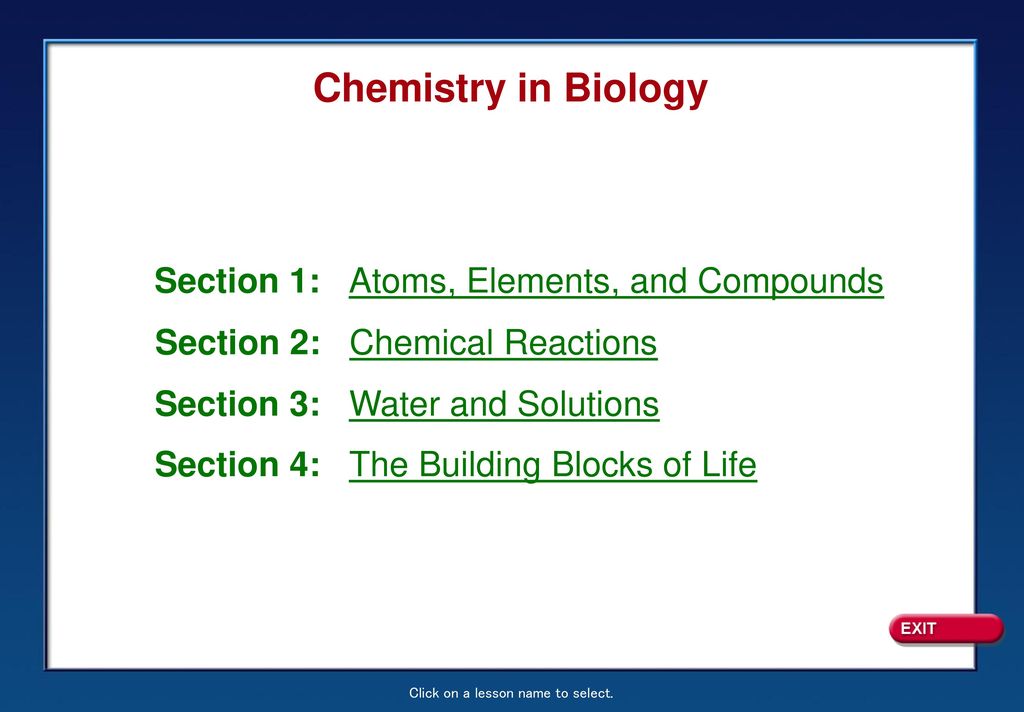
The chemical equation described in section 41 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sidesThis is a requirement the equation must satisfy to be consistent with the law of conservation of matter. Atoms are extremely small typically around 100 picometers across. When a Ca atom loses both of its valence electrons the result is a cation with 18 electrons a 2 charge and an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6.
116 Appendicular Muscles of the Pelvic Girdle and Lower Limbs. Every solid liquid gas and plasma is composed of neutral or ionized atoms. Table 16 Examples of Weak Organic Acids.
Some Applications of d. Versatile nature of carbon. Atoms of different elements differ in mass.
It is a soft silvery-white alkali metalUnder standard conditions it is the least dense metal and the least dense solid elementLike all alkali metals lithium is highly reactive and flammable and must be stored in vacuum inert atmosphere or inert liquid such as. All the atoms of a given element have identical properties including identical mass. 112 Naming Skeletal Muscles.
23 Chemical Reactions. For the printable PDF version of this table with the common polyatomic ions click the link below. This can be explained with the concept of effective nuclear charge Z eff.
113 Axial Muscles of the Head Neck and Back. The atomic nucleus is the small dense region consisting of protons and neutrons at the center of an atom discovered in 1911 by Ernest Rutherford based on the 1909 GeigerMarsden gold foil experimentAfter the discovery of the neutron in 1932 models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Molar Mass and Chemical Compounds.
Modification of work by vxlaFlickr. As shown in Figure 631 as we move across a period from left to right we generally find that each element has a smaller covalent radius than the element preceding itThis might seem counterintuitive because it implies that atoms with more electrons have a smaller atomic radius. An atom is the smallest unit of ordinary matter that forms a chemical element.
Modification of work by the Italian voiceFlickr. They are so small that accurately predicting their behavior using classical physics as if they were tennis balls for example is not possible due to quantum effects. The True Basis of the Periodic Table.
As of 1 February 2022 the EU will continue to publish all EU TBT notifications EU Member States TBT notifications and third country TBT notifications on which the EU decided to send comments. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. It helps EU economic operators to get acquainted with rules applying to products in third countries see section being informed.
22 Chemical Bonds. More on Chemical Compounds. 1 short answer 1 long answer.
A strong base like NaOH also dissociates completely into Na and OH-. By the twentieth century it became apparent that the periodic relationship involved atomic numbers rather than atomic masses. The processes of making and breaking down sugar molecules illustrate two types of metabolic pathways.
There will be no remaining HCl when this happens. Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had. The properties of the elements are periodic functions of their atomic numbersA modern periodic table arranges the elements in increasing order of their atomic numbers and.
The d and f Block Elements. Chapter 2 Atoms Elements and The Periodic Table. 24 Inorganic Compounds Essential to Human Functioning.
Note that when mercury carries a 1 charge it forms an uncommon polyatomic ionic state Hg 2 2 where two Hg atoms share electrons and then each also have a 1 charge state see section XX for more details about polyatomic ions and Hg 2 2. For example magnesium atomic weight 243 is placed to the right of sodium atomic weight 230. Bonding in Carbon The covalent bond.
Sections 64 and 65. Chemical properties of Carbon compounds. Chemical reactions involve reorganisation of atoms.
Molecules are formed when one or multiple atoms combine by chemical bonds. Wood silk bio-based materials eg. Atoms of group 17 gain one electron and form anions with a 1 charge.
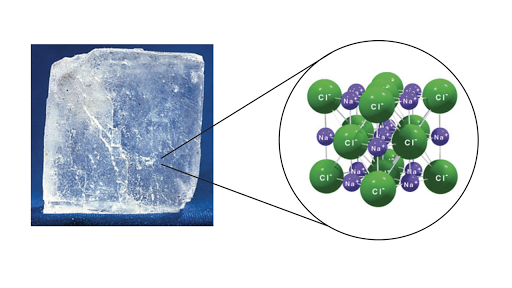
When they are combined on the righthand side they form a single molecule of water H 2 O. Chapter 3 Ionic and Covelent Bonding. Position in the Periodic Table.
Chapter 4 The Shape and Characteristics of Compounds. Upper Panel The Periodic Table of the Elements is an organized chart that contains all of the known chemical elementsLower Panel To the left of the arrow is shown one atom of oxygen and two atoms of hydrogenEach of these represent single elements. Chapter 1 Measurements in Chemistry.
The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. A metabolic pathway is a series of interconnected biochemical reactions that convert a substrate molecule or molecules step-by-step through a series of metabolic intermediates eventually yielding a final product or products. A higher atomic weight than the one on its left.
For example calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. General Properties of the Transition Elements d-Block 84. The broadest definition of a natural product is anything that is produced by life and includes biotic materials eg.
61 Definition and Uses. When atoms of nonmetal elements form ions they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. Image by Aleia Kim.
Some Important Compounds of Transition Elements. Stone is a chemical element with the symbol Li and atomic number 3. 21 Elements and Atoms.
Acid Nomenclature and Summary of Chemical Nomenclature. Chapter 6 A Brief History of Natural Products and. Some important carbon compounds ethanol and ethanoic acid.
Bioplastics cornstarch bodily fluids eg. Student Study Guide Chapter 6. 2 very short answers 3 long answers.
This section explains the atoms and molecules class 9 science notes in detail.

Comparing Elements Compounds Mixtures Definitions Atom Molecule Pure Impure Meaning Physical Change Evidence Of Chemical Change Explained Gcse Ks3 Science Ks4 Igcse O Level Chemistry Revision Notes
Elements Compounds And Molecules Teaching Resources

Chapter 6 Study Guide Key

Atoms Elements And Compounds Chapter 6 Ppt Video Online Download

Section Review Answers Chapter 2 Section 1 1 Matter Has Mass

Compounds In Chemistry Overview Examples What Is A Compound Video Lesson Transcript Study Com

5 4 Mixtures Of Elements And Compounds Atoms Siyavula

Chapter 6 Study Guide Key Name Key Study Guide Date Class Chapter 6 Section 1 Atoms Elements And Compounds In Your Textbook Read About The Course Hero

Understanding Atoms Elements And Compounds Lesson And Worksheets
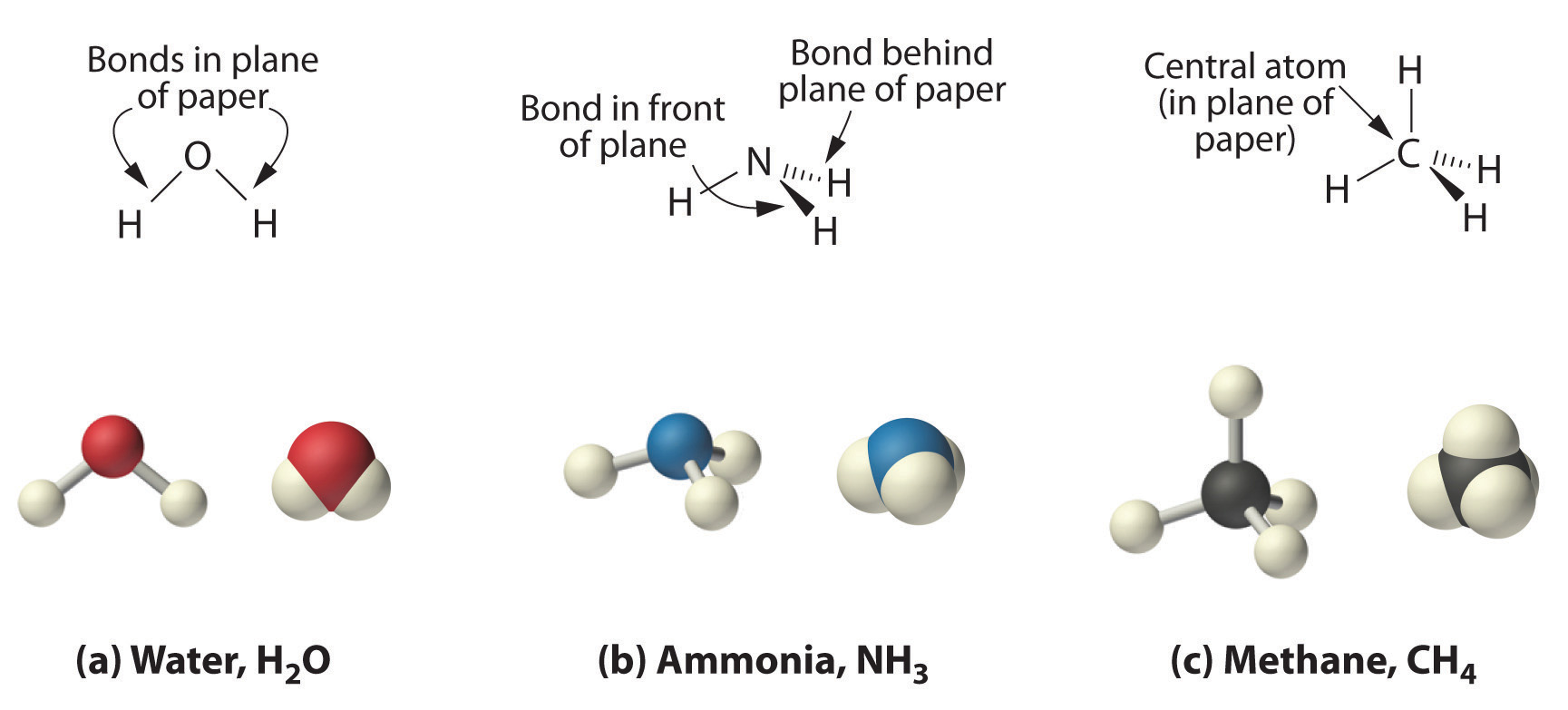
3 1 Types Of Chemical Compounds And Their Formulas Chemistry Libretexts
Chemistry In Biology Section 1 Atoms Elements And Compounds Ppt Download

Elements Atoms Molecules Ions Ionic And Molecular Compounds Cations Vs Anions Chemistry Youtube
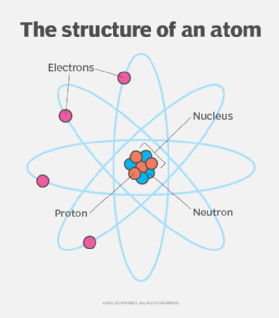
What Is An Element In Chemistry And Computing
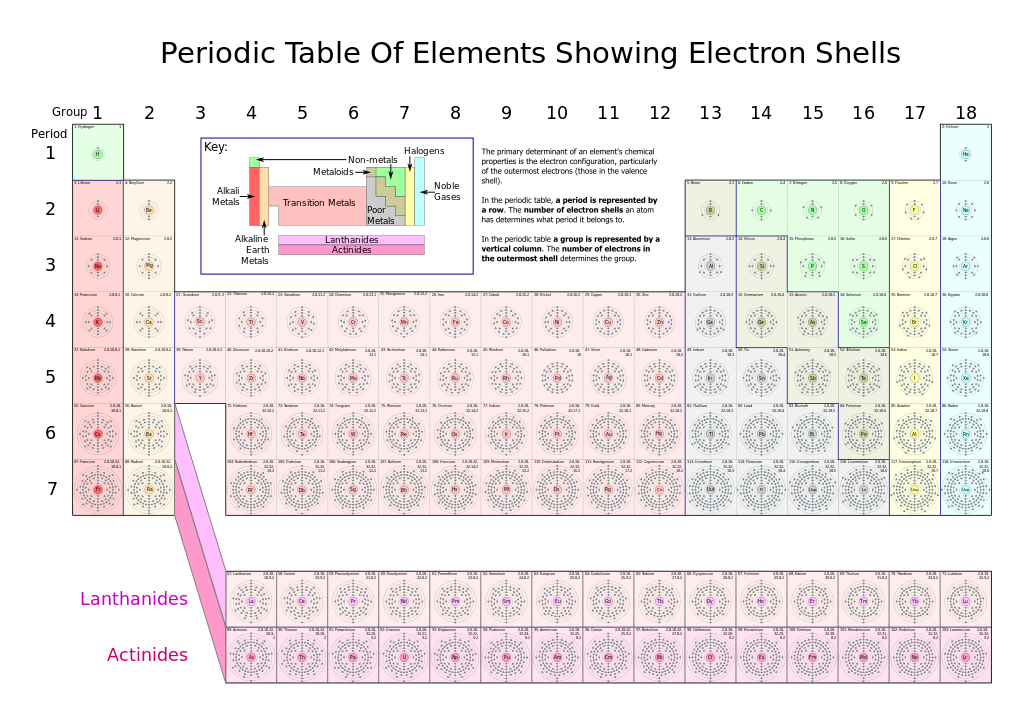
Ch105 Chapter 2 Atoms Elements And The Periodic Table Chemistry

Elements Compounds Mixtures 2 1 1 Cie Igcse Chemistry Revision Notes 2023 Save My Exams

Atoms Elements And Compound Test Study Guide

Chapter 1 Elements And Atoms Bio 140 Human Biology I Textbook Libguides At Hostos Community College Library